FDA Reports Recall of Shoulder Surgery Device
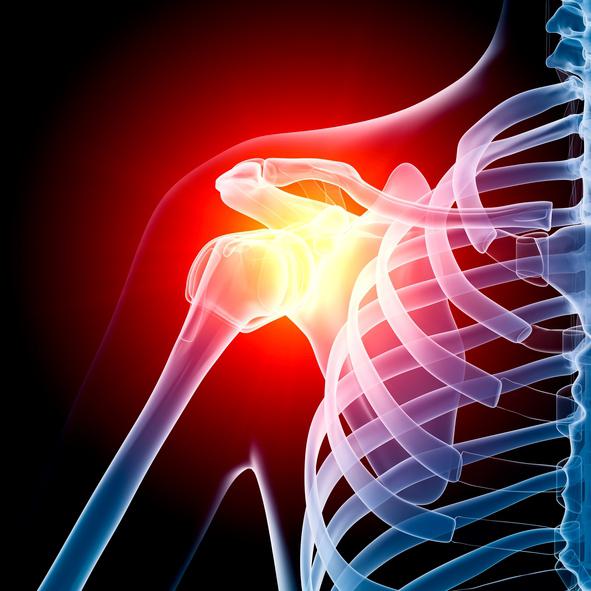
A surgically implanted shoulder replacement device has been recalled due to a high rate of fracture that may lead to serious injury or death, according to an FDA alert. This is a Class 1 recall, the most serious type.
Manufactured by Zimmer Biomet, the Comprehensive Reverse Shoulder is commonly used in surgeries for patients with rotator cuff tears and severe shoulder arthritis, and who failed with previous shoulder joint replacements. This type of device allowed surgeons to treat conditions that had no other solution.
However, when the devices began to fracture more frequently than the label stated, there was concern. Fractures may require another round of surgery, which could result in the permanent loss of movement in the shoulder, or even infection or death. Zimmer Biomet issued the recall on December 15, 2016.
Health providers are asked to return their devices. Patients with these devices implanted will want to seek counsel from their medical provider and/or attorney. “Health care professionals and consumers with questions are instructed to contact the 411 Technical Services by phone at (574) 371-3071 or by email at corporatequality.postmarket@zimmerbiomet.com,” reported the FDA.
Contact us if you have questions or need advice on a particular legal situation.

